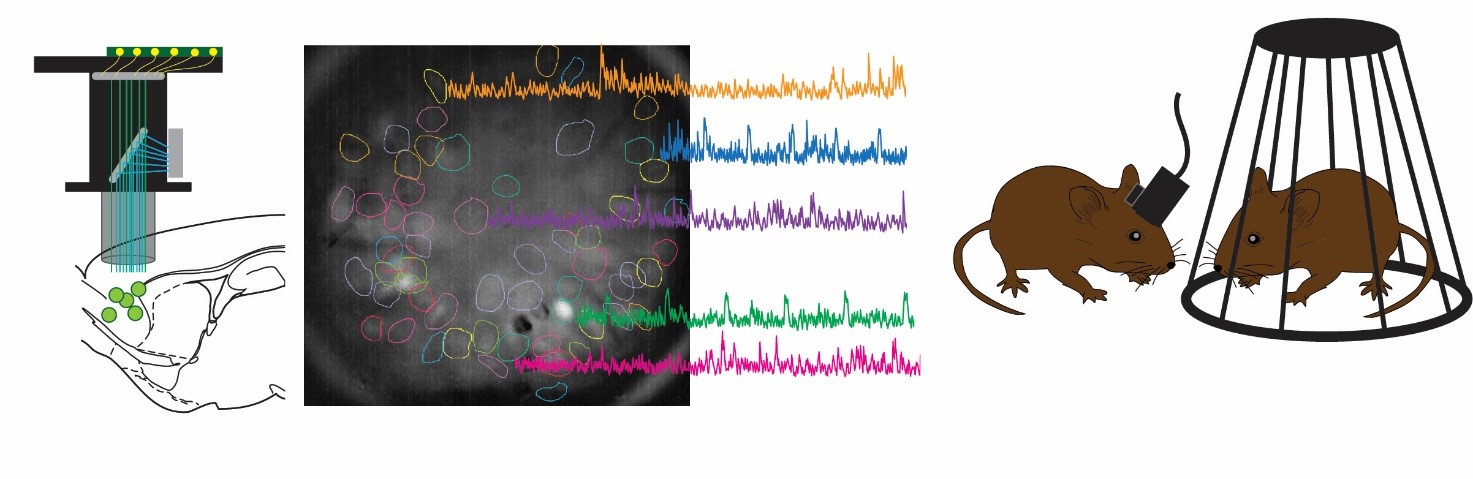
Our lab aims to combine mouse-human behavioural and genetics data, taking advantage of cutting-edge techniques available in rodents to dissect in vivo circuit-, time- and cell-specific mechanisms of cognitive and social processes. Our behavioural, genetics, pharmacological and molecular expertise is integrated with the interdisciplinary environment of the Istituto Italiano di Tecnologia, with state of the art equipment and recognized scientists with diverse backgrounds (e.g. neuroimaging, electrophysiology, molecular biology, nanotechnology, bioinformatics, drug discovery, computation, robotics, smart materials etc.).
Below are some of the main technologies we incorporate into our research to answer our scientific questions: